Describe Experiments to Prepare Insoluble Salts Using Precipitation Reactions
Encounter in this experiment are neutral hydroxides. Precipitation reactions in nature can account for mineral formation in.
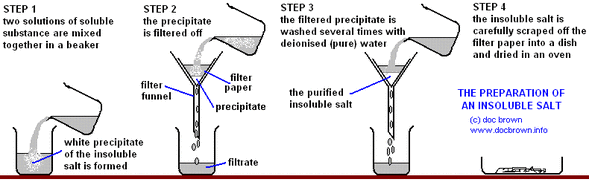
4 8 Describe Experiments To Prepare Insoluble Salts Using Precipitation Reactions Igcse Chemistry Revision Help
Mix silver nitrate and sodium chloride together in a beaker.

. This process is called precipitation. The solubility of a compound depends on the ionic strength of the solution its pH and. Method c Preparing an Insoluble Salt by a precipitation reaction The procedure involves making an insoluble salt my mixing solutions of soluble compounds to form a precipitate.
Precipitation gravimetry is a gravimetric analysis technique that uses a precipitation reaction to calculate the amount or concentration of an ionic compound. Stir the mixture using a. AgNO3aqueous KCl aqueous AgCl precipitate KNO3aqueous In the above reaction a white precipitate.
Add 5 cm3of 1 MleadII nitrate solution to a test tube. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Place a known volume of one soluble salt solution in a beaker.
Experiment to prepare insoluble salts using ppt reactions. Preparation of insoluble lead sulphate PbSO 4. When solutions of two soluble salts are mixed a solid may form.
Designed for students to fill in after watching a. Save the mixture and the remaining solutions of A and B for later use Describe the appearance of the mixture. The solid is called a precipitate and the reaction is called a precipitation reaction.
Add enough distilled water about 20 mL or less to dissolve the salt. The meaning of the terms insoluble and precipitateSolubility Rules and How to Use a Solubility Table Experiment 184 - Preparing an insoluble salt by precipitation. Add the 110 volume of sodium acetate.
But when two ions find each other that form an insoluble compound they suddenly fall. Pour this solution into a 100 mL volumetric flask if available or a 100 mL graduated cylinder. For plasmid DNA or low concentrate DNA use this DNA precipitation protocol.
Up to 24 cash back Add dilute sodium chloride solution to dilute silver nitrate solution until no more precipitate forms. Leave the residue to dry on filter paper. Adding more OH1-causes some insoluble hydroxides to re-dissolve.
48 describe experiments to prepare insoluble salts using precipitation reactions. Up to 24 cash back needed salt. Sodium Chloride Silver Nitrate --.
Precipitation reactions are used to make. Titration must be used if the reactants are soluble. Choose 2 suitable soluble salts eg.
If a centrifuge was used add a little more purified water to the test tube then. This will vary slightly but all students should obtain a purple precipitate. Filter to collect residue.
Add enough distilled water to. Precipitation reactions with equations. Obtain the inorganic salt whose cation and anion you must identify.
Salts need to react together in a precipitation reaction. Prepare the sodium acetate solution of 05M at pH 52. Insoluble salts are made by precipitation reactions.
To make an insoluble. The chemical equation for this precipitation reaction is provided below. For example AlOH 3 s reacts to form the complex ion AlOH 4.
Precipitation is a technique used to separate a mixture based on the solubility of its components. The table shows soluble and insoluble salts. Keep adding the other soluble salt solution and stir to mix until no more precipitate forms.
Conduct preliminary tests for the anion group-wise until you obtain a. Worksheet aimed at GCSE students looking at how to make an insoluble salt from two soluble salts by precipitation. Wash the residue with cold distilled water.
A lot of ionic compounds dissolve in water dissociating into individual ions. 5 Soluble salts can be made by reacting an acid with a metal hydroxide a metal oxide or a metal carbonate. Up to 24 cash back 1.
Fe 2 aq 2 OH aq Fe OH2 s Al 3 aq PO 43 aq AlPO 4 s Minerals are water-insoluble compounds. Edexcel GCSE Science new specification 2011 practical experiment sheet with instructions to fill in while carrying out precipitation reactions. Insoluble salts can be made by using a precipitation reaction.
Step-by-Step Process for Salt Analysis. Then add an equal voStage 21.

Gcse Chemistry Making An Insoluble Salt By Precipitation Youtube
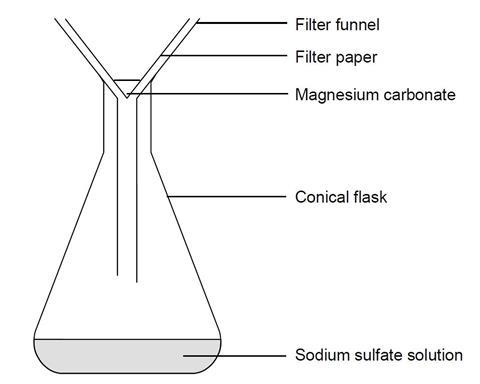
Making Magnesium Carbonate The Formation Of An Insoluble Salt In Water Experiment Rsc Education

How To Make Insoluble Salt Precipitation Method Salt Preparation Online Video O Level Secondary Chemistry Tuition

No comments for "Describe Experiments to Prepare Insoluble Salts Using Precipitation Reactions"
Post a Comment